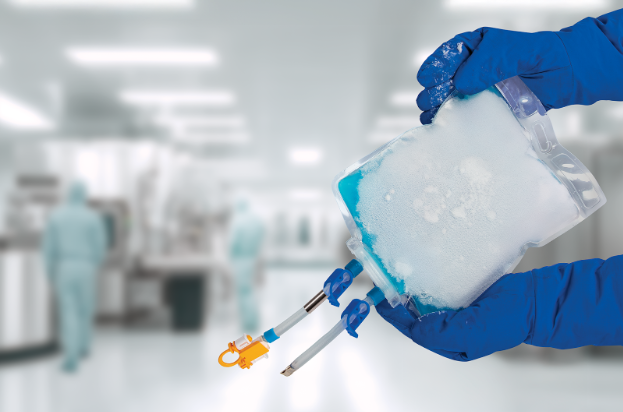
Meissner has announced the extension of its TepoFlex® biocontainer platform to include freeze and thaw applications, bringing a proven single-use solution into low-temperature biopharmaceutical workflows.
The TepoFlex® polyethylene film platform has earned industry trust through nearly two decades of commercial use. Building on this established performance, the biocontainers are engineered to maintain strength, integrity and leak resistance throughout demanding freeze and thaw cycles, increasing their suitability for drug substance and drug product handling, storage and transport.
Designed for freezing temperatures down to -80°C, the 2D TepoFlex® biocontainers are available as single-use options in volumes ranging from 50 mL to 10 L. This range enables manufacturers to select container sizes that align precisely with process scale and operational requirements.
The flexible design of the biocontainers allows straightforward integration into existing freeze and thaw workflows. Compatibility with both blast freezers and vertical plate freezers ensures they can be deployed across development, clinical and commercial manufacturing environments without significant infrastructure changes.
For biopharmaceutical manufacturers seeking a secure, adaptable single-use solution capable of protecting sensitive materials under freeze and thaw conditions, the TepoFlex® biocontainer offers a reliable option.
All freeze and thaw biocontainers are produced in ISO-certified cleanroom facilities using qualified components to support biocompatibility and regulatory compliance. Meissner’s Applications Engineering Team provides expert support to assist customers with system design and optimisation, ensuring assemblies are configured to meet specific application needs.
With over 40 years of experience serving the life sciences sector, Meissner focuses on building long-term partnerships by understanding client challenges and delivering consistent, high-quality customer support.
To discover why leading biopharmaceutical manufacturers trust TepoFlex® for their most essential freeze and thaw applications, visit Meissner’s website at https://www.meissner.com/tepoflex-freeze-thaw where technical resources are available for download.
Additionally, visiting https://www.meissner.com/applications/freeze-thaw/ provides an overview of all of Meissner’s single-use freeze and thaw platform offerings.
About Meissner
Meissner manufactures advanced microfiltration products and therapeutic manufacturing systems for critical pharmaceutical and biopharmaceutical applications, such as sterilization of injectable drugs, and the development and manufacture of life-enhancing/life-saving drugs, therapeutics, biologics, and cell and gene therapies.
Meissner’s manufacturing campuses include a headquarters location in Camarillo, California, USA, a European manufacturing location in Castlebar, Ireland, and buildout of a $250 million facility in Athens, Georgia.
Meissner’s product portfolios are built upon a solid foundation of quality, operational excellence, and technical expertise for delivery of high-performance products that support clients’ critical applications. For more information about Meissner, please visit https://www.meissner.com.